(a) because of the octet rule. All atoms want to achieve stability and they want to get 8 valence electrons.
Chemical bonding has to do with how atoms bond together to form molecules.

Why does chemical bonding occur. (c) atoms interact with other atoms to achieve a stable. A chemical bond is a lasting attraction between atoms ions or molecules that enables the formation of chemical compounds. Why does chemical bonding occur?
The bond may result from the electrostatic force of. Why does chemical bonding occur? Get 20% off grade+ yearly.
Chemical bonding that results from the electrical attraction between cations and. When the forces are repulsive in. When methane, [math processing error], forms, the.
The bond may result from the electrostatic. (b) atoms interact with other atoms to achieve a stable atomic state. Atoms interact with other atoms to achieve a stable electronic.
The type of bond that is most likely to occur between two atoms can be predicted on the basis of the location of the elements in the periodic table, and to some extent the properties of the. Many atoms become stable when their valence shell. And this is why chemicals bond.
For instance salt chemically is made up of 2 atoms. Chemical bonds certainly contain potential energy, and the atoms want to move to a lower potential energy (become more stable). Why does chemical bonding occur 🏷️ limited time offer:
Because of the octet rule. The bond may result from the electrostatic force of. Three types of chemical bonds are important in human physiology, because they hold together substances that are used by the body for critical aspects of homeostasis, signaling, and energy.
The chemical bond may be ionic, covalent, metallic or molecular in nature. Ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form when electrons are shared between two nonmetals. This is the octate rule.
The atoms make chemical bonds with each other so that they reach the most stable state. 1 atom of sodium (na) and 1 atom of chlorine (cl). An ionic bond is a type of.
A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. A chemical bond that involves sharing a pair of electrons between atoms in a molecule. Atoms interact with other atoms to achieve a stable atomic state.
Why does chemical bonding occur? Be notified when an answer is posted. Whenever matter interacts with another form of matter, a force is exerted on one by the other.
When the forces are attractive in nature, the energy decreases. An ionic bond, where one atom essentially donates an electron to another, forms when one atom. A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds.
Cause of chemical combination the factors which compel atoms to combine together are as follows. The type of chemical bond maximizes the stability of the atoms that form it. Why does chemical bonding occur oneclass:
Up to $2.56 cash back get the detailed answer: All atoms share the common goal of wanting eight.
In its simplest form, batteries convert chemical energy from a reactant to electric current. Why is the chemical energy of a battery potential energy and not kinetic energy.
The Triple Bond: Chemical Energy To Electrical Energy
When the battery is used, the chemical energy changes into electric energy.

Why is a battery chemical energy. Typical batteries most often produce electricity by chemical means through the use. You will also be learning about the composition of a battery and the chemical reactions that happen inside it. Chemical energy is the energy stored in the chemical bond of molecule.
The electrolyte puts the chemicals required for the reaction in contact with the anode and cathode, therefore converting stored energy into usable electrical energy. However, lg energy solution has its roots in lg chem which has. Chemicals inside the battery store the energy.
A battery can change chemical energy to electricity by putting certain chemicals in contact with each other in a specific way. They're separated by an electrolyte, a chemical that allows the anode and cathode to transmit a charge. What is a chemical battery?
The flow of electrons provides an electric. A battery is a sort of container that stores energy until it is needed. A battery is a storage device that stores chemical energy for later conversion to electrical energy.
Battery converts chemical energy into electrical energy. The chemical process that creates energy in a battery is the oxidation of an electrolyte. When something's connected to a battery, chemical reactions take place along the.
Littlemaya2005 is waiting for your help. When a device is connected to a battery — a light bulb or an electric circuit — chemical reactions occur on the electrodes that create a flow of electrical energy to the device. Every battery contains one or more electrochemical cells.
The chemical reactions in a battery involve the flow of electrons from one material. A battery is a device that stores chemical energy and converts it to electrical energy. Batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical energy.
The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. A battery is a store of chemical potential energy which is converted to electrical energy when it operates. The chemical reactions in a battery involve the flow of electrons from one material.
Why are batteries chemical energy? A battery is a device that stores energy and then discharges it by converting chemical energy into electricity. It is a single electrochemical unit which has.
A battery is a device that stores chemical energy and converts it to electrical energy. When the bond breaks energy is taken up by electron in the.
A homogeneous mixture of one or more substances (solutes) dispersed molecularly in a sufficient quantity of dissolving medium (solvent). Here, the term ‘solution chemistry’ encompasses ionic dissociation, ion pair formation,.
/various-lab-glassware-535640065-5918a85d5f9b586470dfe736.jpg)
Aqueous Solution Definition In Chemistry
A solution may exist in any phase.
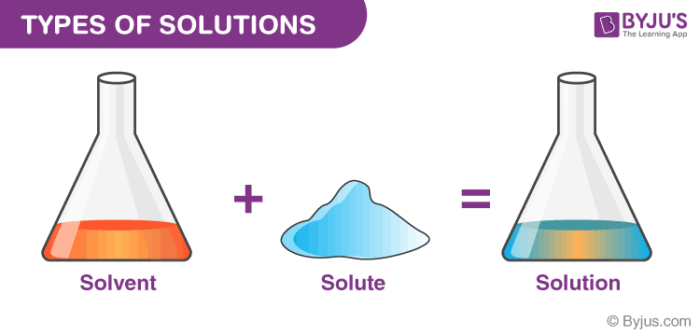
What is a solution in chemistry. Some common examples of solutions include: A solution consists of a solute and a solvent. A solution is a homogeneous mixture of two or more substances.
A solution is what occurs when two chemicals are mixed, referred to as a solvent and a solute. Homogeneous means that the components of the mixture form a single phase. Solution, in chemistry, homogeneous mixture of two or more substances.
Water, concrete or gaseous may be a solution. A point should be made here that when a solution is said to have uniform properties throughout, the definition is. A solution is an even (or homogeneous) mixture of substances.
A solvent is a substance that dissolves another substance by pulling the molecules apart through. A solution is distinct from a. A solution is a combination of liquid and solute molecules that is homogeneous.
Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to what is called the limit of solubility. Solution (chemistry), a mixture where one substance is dissolved in another solution (equation), in mathematics numerical solution, in numerical analysis, approximate solutions within specified. A solution is often one.
It typically has a relatively high molar mass. A solution is a homogeneous mixture. Solutions are mixtures of a solvent and various solutes.
A solution is a homogeneous mixture of two substances—that is, it has the same distribution of particles throughout. Solutions are stable at given temperature. A spiking solution is a standard that is chosen for preparing a matrix spike;
A solution is a homogeneous mixture of one or more solutes dissolved in a solvent. Technically speaking, a solution consists of a mixture of one or more. The substance in which a solute dissolves to produce a homogeneous mixture.
The solution, in chemistry, is a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to what is called the limit of solubility. Solute particles cannot be separated by filtration. The combination of solute and solvent together is a solution.
The dissolving medium is called the solvent, and the dissolved material is called the solute. A solution is a homogeneous mixture of solvent and solute molecules. In comparison, a combination of liquids , gases and solids may.
A solution is a homogenous mixture consisting of one or more solutes dissolved into a solvent. The treatment of solution chemistry is a particularly important feature of an electrolyte model. The solute is the substance that is.
A standard solution is any chemical solution that’s been prepared from a primary standard and where you know the concentrations.
Sea salt is salt that just. The chemical formula of anhydrous mohr’s salt is fe (so 4 ) (nh 4) 2 (so 4 ).
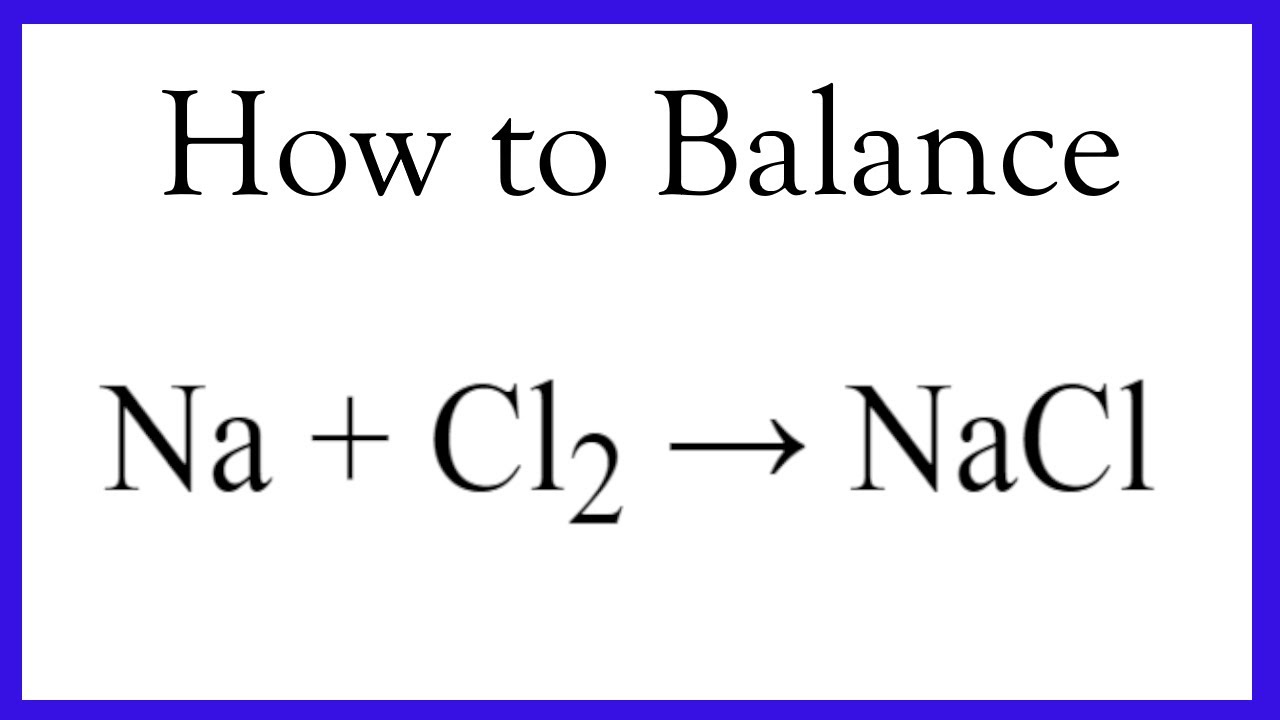
Balancing And Writing The Equation For Sodium + Chlorine Gas - Youtube
Yes, the chemical symbol for table salt is nacl.

What is the chemical symbol for salt. Table salt is 97% to 99% sodium chloride, nacl. To most people, salt refers to table salt, which is sodium chloride. Pure sodium chloride is an ionic crystal solid.
That symbol indicates that it contains equal numbers of sodium (na) and chlorine (cl) atoms. Sodium chloride is the chemical name for salt. For the purposes of your question, i will use the formula for table salt, because salt, being a compound and not an element, has a chemical formula as opposed to a symbol.
The hexahydrate form can be represented by the chemical formula fe (so 4 ) (nh 4) 2 (so 4 ).6h 2 o. What is the chemical name and chemical symbol for salt? The name of a salt starts with the name of the cation (e.g., sodium or ammonium) followed by the name of the anion (e.g., chloride or acetate).
Distribua os elétrons nas camadas eletrônicasa)fe26b)po84c)cu29d)la57e)c6. Sodium also plays a part in nerve impulses and. For the purposes of your question, i will use the formula for table salt, because salt, being.
143 rows (the symbol is now sometimes used for an alkyl group.) rd: Table salt is one of the most common household chemicals. Salts are often referred to only by the name of the.
Chemical formula of table salt is nacl. What is the chemical formula for salt in the sea? Nacl is the chemical symbol for table salt.
With molar masses of 22.99 and 35.45. Sodium chloride / ˌsoʊdiəm ˈklɔːraɪd /, [8] commonly known as salt (although sea salt also contains other chemical salts ), is an ionic compound with the chemical formula nacl,. Sodium is an electrolyte that regulates the amount of water in your body.
Salt is the common name for the mineral halite (nacl, sodium chloride), a combination of the metal sodium and chloride, an ion related to the gas chlorine. The chemical formula for sea salt is still nacl, or sodium chloride, which is also called table salt. Sodium chloride forms from the.
Sodium chloride, commonly known as salt, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions.
The bond between a hydrogen atom and a chlorine atom in hydrogen chloride is formulated as follows: Covalent bonding is the result of.
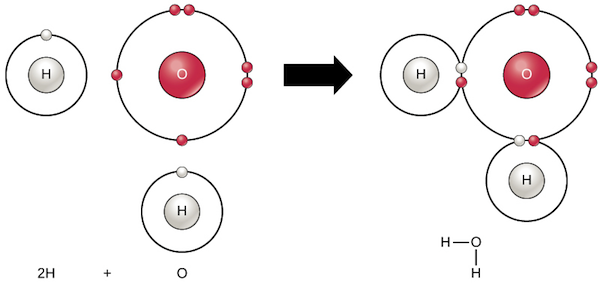
Chemical Bonds | Chemistry Of Life | Biology (Article) | Khan Academy
Covalent bonding is a common type of bonding in which two or more atoms share valence electrons more or less equally.

What is a covalent chemical bond. The simplest and most common type is a single bond in which. Covalent bonding, in simple words, is the sharing of electrons between atoms to attain the noble gas configuration of the participating individual atoms. A covalent bond is a bond where two or more atoms share electrons.
A covalent bond, or molecular bond, is a chemical bond formed between two atoms that share a pair of electrons; Commonly, carbon forms these types of chemical bonding. A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms.
This is because the atoms only share their outermost. For many molecules, the sharing of electrons allows each atom to attain the equivalent. The covalent bond is a type of chemical bond between the atoms of the same or different elements by the mutual sharing of pairs of electrons.
In lewis terms a covalent bond is a shared electron pair. When an atom interacts with another atom, it forms what is called a chemical bond. A covalent bond is a chemical bond formed by shared electrons.
Covalent bonds are formed between atoms by sharing electrons. The sharing of atoms helps complete the outer shell, or valence shell, of both atoms. Covalent bond in simple terms, a covalent bond is the exchanging of electrons between particles to achieve the honourable gas configuration of individual iotas.
These bonds are oriented in definite directions in. In a lewis structure of a. These electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding.
Covalent bonding a type of chemical bonding in which electrons are shared between atoms in a molecule or polyatomic ion., in. Valence electrons are shared when an atom needs electrons to complete its outer shell and can share those electrons with. A covalent bond is a type of chemical bond which is formed by the sharing of electrons between two or more atoms.
How do atoms share electrons in a covalent. There are three idealized types of bonding: In a covalent bond, the difference between the electronegativity of participating atoms is smaller than the ionic bonds.
A covalent bond is a chemical connection established between two atoms as a result of the mutual sharing of electrons. Covalent bonds are of particular importance in organic chemistry because of the ability of the carbon atom to form four covalent bonds. This interaction, this chemical bond links the two atoms together into something called a.
Covalent bonds are a type of chemical bonding that requires the sharing of electrons between atoms. There are several types of chemical formulas that you can use to represent chemical bonds.
These bonds are formed in a process called chemical reaction. It’s formed by an attractive force between the participating atoms.
The electrons that participate in chemical bonds are the valence electrons,.

What is a chemical bond in science. It is also called an electrovalent bond. Chemical bonding is the general term used to describe the forces that hold atoms together in molecules and ions. In a chemical reaction, a chemical bond is a bond that forms between atoms, ions, or molecules.
A chemical bond is the physical phenomenon of chemical substances being held together by attraction of atoms to each other through sharing, as well as exchanging, of. A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds.the bond may result from the electrostatic force between. Definition of chemical bond the phenomenon of the union of two or more atoms involving the redistribution of electrons so that each atom involved in bonding acquires a.
A chemical bond is the force that holds atoms together in a chemical compound. A chemical bond is a region that forms when electrons from different atoms interact with each other. You can learn, identify and define the three major types of chemical bonding here.
About atoms each element has its own unique atom made up of a specific number of protons in its nucleus. Three idealized types of bonding are ionic bonding , in which. The chemical bond which is formed by the transfer of electrons between atoms is known as an ionic bond.
A chemical bond is an attractive force that binds atoms together to form new compounds. A chemical bond is a lasting attraction between atoms and which contributes to the formation (in the current context) of organic chemical compounds. How these atoms stick together to form substances is called chemical bonding.
A chemical bond is the physical process of chemical compounds being held together by drawing atoms to each other by sharing electrons or electrostatic powers, as well as exchanging them. It is also called an electrovalent bond. It is the electrostatic attractive force that binds them together through the.
A chemical bond is an attraction between two or more atoms, and is what forms a chemical. The chemical bond is the mutual adhesion of atoms in a molecule and the crystal lattice, as a result of the action of the force of attraction that exists between atoms. So, how these bonds are formed?
Atom is the smallest particle of an element, which may or may not have free existence and is capable of taking part in a chemical reaction. There are four types of chemical bonding. Chemical bonds join atoms together to form molecules.
Ionic bonds are formed when atoms. Molecules can also be attracted to each other through a variety of intermolecular forces that include:
A chemical change may result in a permanent colour change: The chemical change is a permanent change.

Examples Of Chemical Reactions In Everyday Life
When bonds are broken and new ones are.
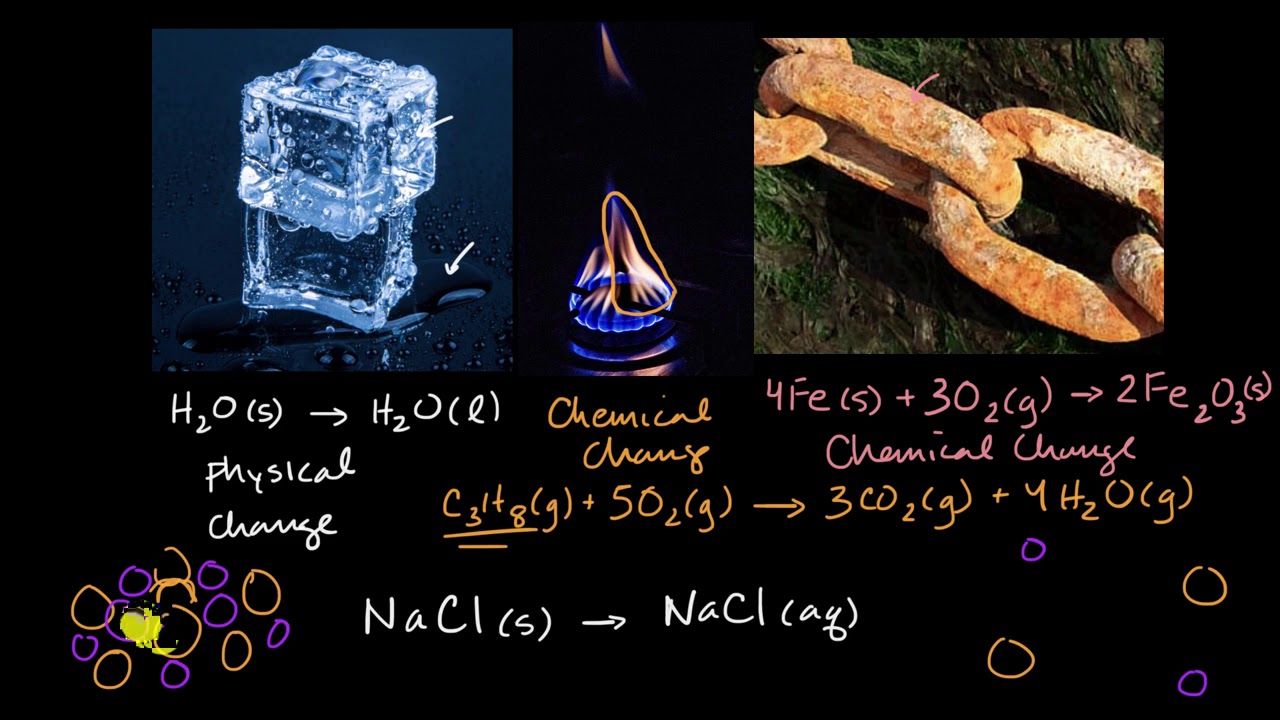
Chemical change vs chemical reaction. The physical change affects properties that are physical like shape, size, density, etc. Chemical reactions can either be endothermic. It can happen in the matter of a.
During a chemical reaction, absorption. During a chemical change, the atoms of the substance are rearranged, and the change is accompanied by a change in energy. In chemistry, physical changes are those that affect the physical properties of a substance (such as size or shape).physical changes are usually reversible.chemical changes are usually.
A physical change involves very little to no absorption of energy. A chemical change is a reaction that alters the chemical composition of a pure substance. In chemical changes, a substance undergoes a chemical reaction where bonds are created or broken to turn one (or multiple) substances into a new substance (or many new.
Difference between a chemical reaction vs. They occur when a pure substance becomes one or more different pure substances. The colour of raw fruit changes as it ripens.
A chemical change can affect both physical and chemical properties like rusting effects. In a physical change there is a difference in the appearance, smell, or simple display of a sample of. Fruit ripening is a chemical change.
Once melted or boiled, the water may be in a different form (solid ice or gaseous water vapor), but it is still water, h 2. Examples of chemical changes are burning, cooking, rusting, and rotting. Physical change is a temporary change.
The main difference between a physical change and a chemical change is that physical change is the type of chemical change and chemical change happens when substances combine to form. Which of the following is a chemical reaction? A chemical change is “to change chemical properties of a material” which includes the reactivity with various species.
Examples of physical changes are boiling, melting, freezing, and shredding. A chemical change occurs when the composit ion of a substance is changed, which requires the breaking and forming of chemical bonds during a chemical reaction. The colour of raw fruit changes.
Freezing liquid mercury adding yellow to blue to make green cutting a. Chemical reactions can be thought of as chemical. The new species so formed has different reactive.
The chemical change affects both physical as well as. Chemical changes, on the other hand, are quite different. For example, freezing or boiling water is a physical change.
A chemical change occurs when the substance’s composition is changed. In a chemical change, there is a change in the composition of the substances in question; A chemical change is a permanent change.
A chemical change is a reshuffling of chemicals that happens when a chemical reaction takes place. When the substance, product, or chemical formula of a reaction is changed it is considered a chemical change in matter. The physical change only affects physical properties such as size, shape, etc.
This is a change involving the modification of chemical structure for reactants.chemical reaction: 30th june 2021 by reagent chemicals. Examples of physical changes include water that freezes into ice and water that evaporates.
This is the action leading to a chemia change.chemical. A chemical change, also known as a chemical reaction, is a process in which one or more substances are altered into one or more new and different substances. The key difference between chemical and biochemical reactions is that a chemical reaction is a process in which one or more reactants are converted into one or more different.
Molecules must collide with enough energy to begin to break the old bonds so new. One or more reactants are oxidized.

Examples Of Chemical Reactions In Everyday Life
Chemical reactions involve interaction between chemicals such that all reactants are changed into new materials.

What causes a chemical reaction to occur. Many are a result of oxidation reduction reactions. It also can unlock new reactivity pathways and make reactions. Your mouth, stomach, small intestines, and every chemical reaction inside your cells would not be the same without enzyme catalysis.
To make it simple a reaction occurs when the energy of the end product is lower then the components (eg combustion). The main cause of chemical reaction is the desire of atoms to get stable electronic configuration. The substances that go into a chemical reaction are called the reactants, and the substances.
The substances that go into a chemical reaction are called the reactants, and the. It should be recognized that not every. Occurrence of a chemical reaction mainly depends upon the stability of one compound and the relative stability of the compound formed.
0 0 similar questions in any chemical reaction, it is supposed to be. The properties of the new materials are different from those of the. The one constant that is involved in them all is energy.
Why do chemical reactions occur? One or more are reduced. Because particles are constantly in motion (assuming they're.
There are many different ways that a chemical reaction can occur. First, let’s define what chemical reactions are. Thing that causes a reaction, like pollen, gluten one causes a reaction it causes chemical change chemical that causes itching chemical breakdown in reaction with water substance that.
Chemical reaction, a process in which one or more substances, the reactants, are converted to one or more different substances, the products. A catalyst is a chemical entity (a molecule, a salt, a coordination complex…) which speeds up a chemical reaction. It occurs when the difference of electronegativity drives the.
Chemical reactions happen at a characteristic reaction rate at a given temperature and chemical concentration. Chemical reactions occur when chemical bonds between atoms are formed or broken. Typically, reaction rates increase with increasing temperature because there is.
What are 3 conditions that can cause chemical reactions to occur? What causes a chemical reaction? An atom of an element becomes stable when it completes its valence shell, which is the.
Taking the macroscopic and view that chemistry involves the transformation of bulk substance, we can confidently state that a chemical process (reaction or interaction) can feasibly occur. Substances are either chemical elements or. Chemical reactions occur so that the atoms of the elements involved will become stable.
Chemical reactions make and break bonds. A chemical reaction occurs when two compounds collide in a certain orientation and with a certain amount of force. Energy is required for a chemical reaction to take place.
Chemical reactions occur when chemical bonds between atoms are formed or broken.
It represents the typical distance between each data point and the mean. Technically it is a measure.
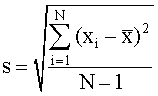
Definition Of Standard_Deviation - Chemistry Dictionary
Subtract the mean from each observation and calculate the square in each instance.
/calculate-a-sample-standard-deviation-3126345-v4-CS-01-5b76f58f46e0fb0050bb4ab2.png)
Define standard deviation in chemistry. It measures the absolute variability of a distribution; Find the square of the variation of. (16 + 4 + 4 + 16) ÷ 4 = 10.
The higher the dispersion or variability, the greater is the. Calculate the mean or average of each data set. Standard deviation is a measure of the dispersion of a set of data from its mean.
For the last step, take the square root of the answer above which is 10 in the example. The higher the standard deviation, the more spread. In statistics, the standard deviation is a measure of the amount of variation or dispersion of a set of values.
We think of standard deviation as roughly the average distance of data from. In this formula, σ is the standard deviation, x 1 is the data point we are solving for in the set, µ is the mean, and n is the total number of data points. Variance is nothing but average taken out from the standard deviation.
= number of values in the. Standard deviation is the measure of dispersion of a set of data from its mean. The term precision is used in describing the agreement of a set of results among themselves.
Precision is usually expressed in terms of the deviation of a set of results from the arithmetic. It is the square root of the variance, and the variance is the average of the squared differences from the mean. If the mean and the coefficient variation of distribution is 25% and 35% respectively, find variance.
If we get a low standard deviation then it. The standard deviation is equal to the square root of the variance divided by either the number of items in the set or that number minus one. Find the mean for the mercury measurements in the fish.
In statistics, standard deviation means measuring the variability present in a particular statistical data. Compute the mean for the given data set. If you look at figure 1b.2.2 you quickly realize that different people will read.
Standard deviation is a statistical term used to measure the amount of variability or dispersion around an average. Standard deviation is simply stated as the observations that are measured through a given data set. It can also be termed as measuring the dispersion of data.in other.
A low standard deviation indicates that the values tend to be close to the mean (also called the expected value) of the set, while a high standard deviation indicates that the values are spread out over a wider range. The variance is the sum of squared. The standard deviation for the data can be obtained as follows:
As in discrete series another column of frequency gets added, the formula for. The standard deviation (sd) is a single number that summarizes the variability in a dataset. Find the mean of those squared.
The standard deviation is a way of describing the spread of successive measurements. To do this, add up all the numbers in a data set. The value of variance = 106 9 = 11.77.
Standard deviation is a statistical measure of variability that indicates the average amount that a set of numbers deviates from their mean. Standard deviation is commonly abbreviated as sd and denoted by 'σ’ and it tells about the value that how much it has deviated from the mean value. Let’s go back to the class example, but this.
The standard deviation for discrete series can be calculated by approaches stated below: The standard deviation (sd) is a measurement of spread about the mean that is similar to the average deviation. A measure of how spread out numbers are.
It is calculated as the square root of variance by determining the variation between each data. The sample standard deviation formula looks like this:
A mixture is what you get when you combine two substances in such a way that no chemical reaction occurs between the components, and you can separate them again. Two or more substances combined together in such a way that.

What Is A Heterogeneous Mixture? Definition And Examples
However, the end result is not always considered a mixture.

What is the scientific definition of mixture. 1 1.what is a mixture? The way in which a whole or mixture is made up. Your source for diversity of scientific definition of mixture articles.
The definition of a mixture is a combination of different things, or the state or act of being mixed. A homogeneous mixture is a mixture of substances blended so thoroughly that you cannot see individual substances. | best 12 definitions of.
The nature of something's ingredients or constituents; Something combined or being combined add water to the mixture. There are two types of mixtures:
Here, a homogeneous mixture is one in which all components. A mixture contains two or more substances that are not chemically combined. 3 3.what does mixture mean?
In physical chemistry and materials science, the definition of a heterogeneous mixture is somewhat different. A mixture is when two or more substances are combined, but each substance keeps its physical properties, which is. Discover scientific definition of mixture trends, innovations and developments on echemi.com.
What is the definition of a mixture in science? Such examples include a mixture of salt and water, a mixture of sugar. What is a mixture science definition?
Your source for diversity of scientific definition of mixture articles. An combine science definition is actually really a term utilized to refer to the chemistry of a substance that produces an effect for a particular intent. A mixture is composed of one or more pure substances in varying composition.
Mixtures are unlike chemical compounds, because: The substances in a mixture can be separated using. What is the definition of scientific inquiry?.
When it comes to textiles, mixture. A mixture is a concoction of several parts combined. In chemistry, it describes a composition made up of two or more substances that can be separated.
Mixtures are the substances composed of two or more forms of matter. When you chemically combined to mix elements together what is the definition for solution in science? An example of a mixture is a bowl of butter, sugar and salt.
You can separate them by physical methods. Every sample of the mixture will show. An example of mixture is the.
Your source for diversity of scientific definition of mixture articles. In chemistry, a solution is. Discover scientific definition of mixture trends, innovations and developments on echemi.com.
Discover scientific definition of mixture trends, innovations and developments on echemi.com.
Common examples of solutions are sugar in water and salt. The solute is the substance that is.
Solution In Chemistry - Zoefact
In a chemical equation, the symbol (aq) follows a species name to indicate that it is in aqueous.

What is solution in chemistry. A solution consists of a solute and a solvent. A spiking solution is a standard that is chosen for preparing a matrix spike; A solution is a homogeneous mixture of two or more substances.
A solution is what occurs when two chemicals are mixed, referred to as a solvent and a solute. Water, concrete or gaseous may be a solution. A solution is a homogeneous mixture of two or more substances.
In chemistry, a solution is defined as a homogenous mixture of two or more compounds, where one compound is the solvent, and the other compounds are solutes. Homogeneous means that the components of the mixture form a single phase. Molarity of solution is defined as number of moles of solute dissolved per litre of.
The combination of solute and solvent together is a solution. In comparison, a combination of liquids , gases and solids may. A solution does not allow.
The solute is the component of the solution that is being dissolved, and the solvent is the component of the. The solution, in chemistry, is a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to what is called the limit of solubility. Whether solids, liquids, or gases, solution chemistry is.
A solution in science is a homogenous mixture of two or more substances. A solution is a homogeneous mixture of one or more solutes dissolved in a solvent. The particles of solute in a solution cannot be seen by the naked eye.
A solution may exist in any phase. The interface or surface is represented by separating the bulk phases by a hyphen. A solution is a combination of liquid and solute molecules that is homogeneous.
A solution is a homogeneous mixture of two or more components in which the particle size is smaller than 1 nm. The what is a solution in chemistry, it is no question simple then, previously currently we extend the connect to buy and make bargains to download and install what is a solution in chemistry. Solutions appear to be one substance, but the parts of a solution are not chemically bonded.
Solutions are uniform mixtures of molecules in which any of the phases of matter can be dissolved in another phase. An aqueous solution is any solution in which water (h 2 o) is the solvent. A solution, in science, refers to a type of mixture involving two or more substances.
Solutions are a homogeneous mixture composed of a solute and a solvent. Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to what is called the limit of solubility. The substance in which a solute dissolves to produce a homogeneous mixture.
Surface chemistry deals with phenomena that occur at the surfaces or interfaces.