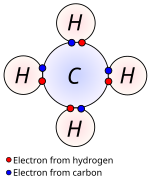
These bonds are formed in a process called chemical reaction. It’s formed by an attractive force between the participating atoms.
The electrons that participate in chemical bonds are the valence electrons,.

What is a chemical bond in science. It is also called an electrovalent bond. Chemical bonding is the general term used to describe the forces that hold atoms together in molecules and ions. In a chemical reaction, a chemical bond is a bond that forms between atoms, ions, or molecules.
A chemical bond is the physical phenomenon of chemical substances being held together by attraction of atoms to each other through sharing, as well as exchanging, of. A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds.the bond may result from the electrostatic force between. Definition of chemical bond the phenomenon of the union of two or more atoms involving the redistribution of electrons so that each atom involved in bonding acquires a.
A chemical bond is the force that holds atoms together in a chemical compound. A chemical bond is a region that forms when electrons from different atoms interact with each other. You can learn, identify and define the three major types of chemical bonding here.
About atoms each element has its own unique atom made up of a specific number of protons in its nucleus. Three idealized types of bonding are ionic bonding , in which. The chemical bond which is formed by the transfer of electrons between atoms is known as an ionic bond.
A chemical bond is an attractive force that binds atoms together to form new compounds. A chemical bond is a lasting attraction between atoms and which contributes to the formation (in the current context) of organic chemical compounds. How these atoms stick together to form substances is called chemical bonding.
A chemical bond is the physical process of chemical compounds being held together by drawing atoms to each other by sharing electrons or electrostatic powers, as well as exchanging them. It is also called an electrovalent bond. It is the electrostatic attractive force that binds them together through the.
A chemical bond is an attraction between two or more atoms, and is what forms a chemical. The chemical bond is the mutual adhesion of atoms in a molecule and the crystal lattice, as a result of the action of the force of attraction that exists between atoms. So, how these bonds are formed?
Atom is the smallest particle of an element, which may or may not have free existence and is capable of taking part in a chemical reaction. There are four types of chemical bonding. Chemical bonds join atoms together to form molecules.
Ionic bonds are formed when atoms. Molecules can also be attracted to each other through a variety of intermolecular forces that include:
Chemical Bond - Examples, Body, Used, Water, Type, Form, Energy, System, Oxygen
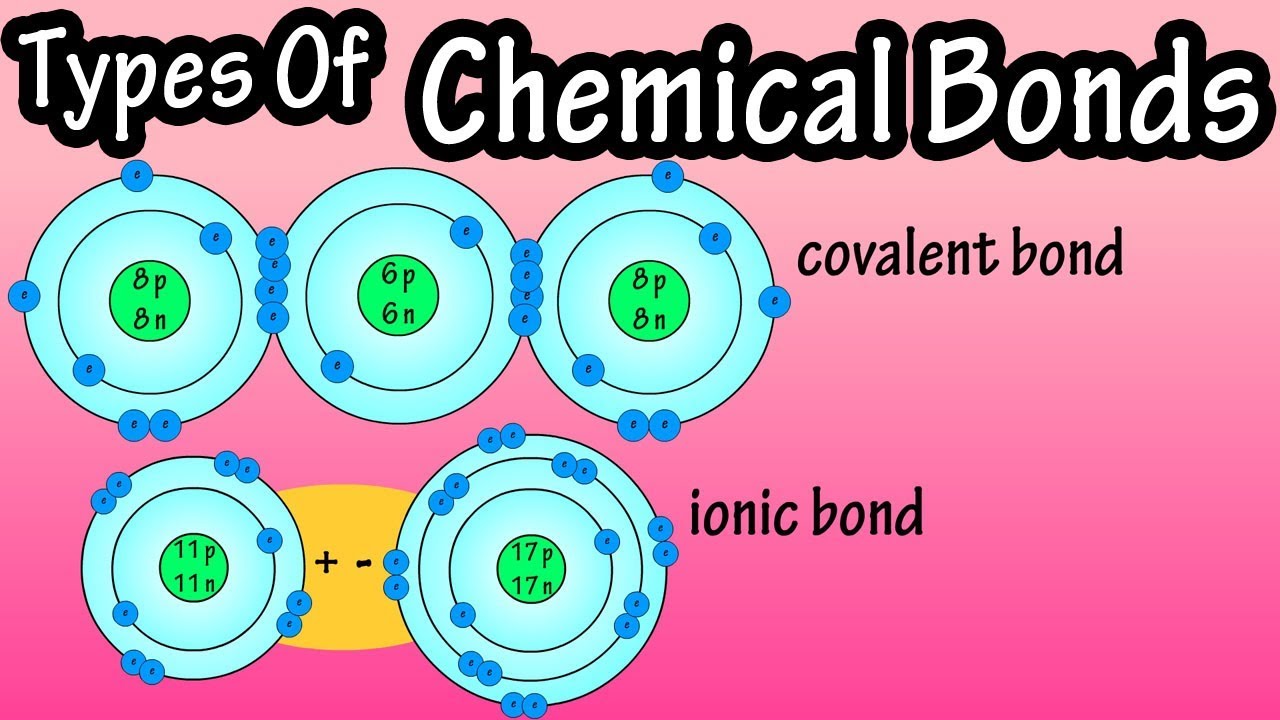
Types Of Chemical Bonds - What Are Chemical Bonds - Covalent Bonds And Ionic Bonds - What Are Ions - Youtube

Covalent Bond | Definition, Properties, Examples, & Facts | Britannica
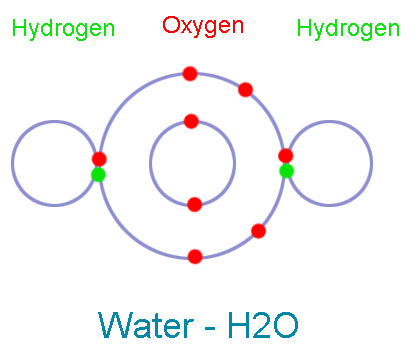
Chemical Bonding: (Types, Formation, And Facts) - Science4Fun
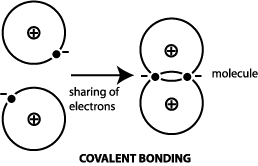
Ces Information Guide - Materials Science Engineering
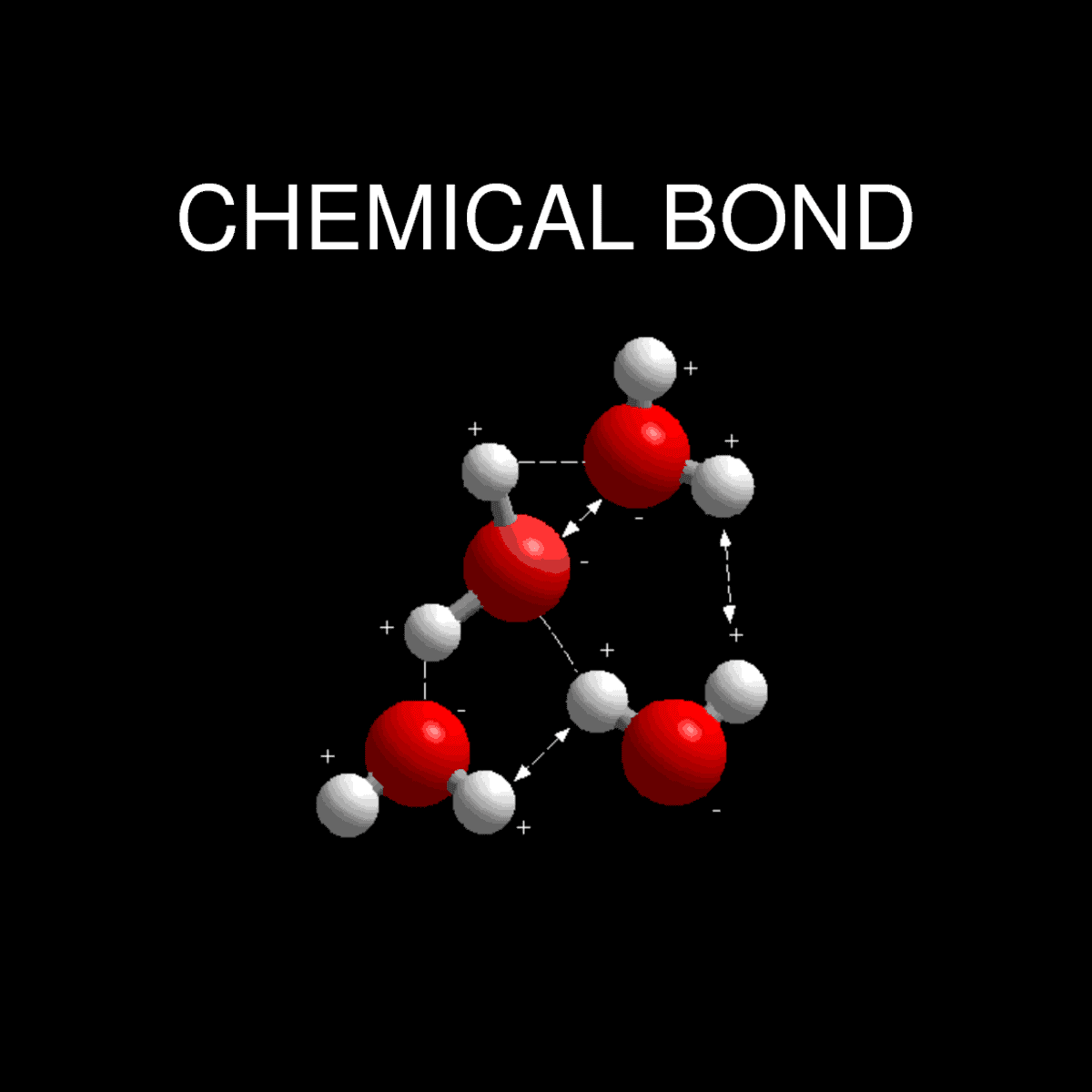
Chemical Bonding: How Do Atoms Combine? What Are The Forces That Bind The Atoms Together? - Owlcation

Chemical Bonding - Ionic Vs. Covalent Bonds - Youtube
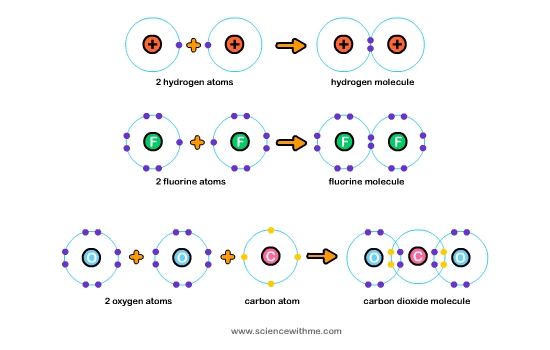
Science With Me - Learn About Bonding

Physical Science Chapter Ppt Download

Chemical Bonds: Definition, Types, And Examples

Chemical Bonding | Definition, Types, & Examples | Britannica
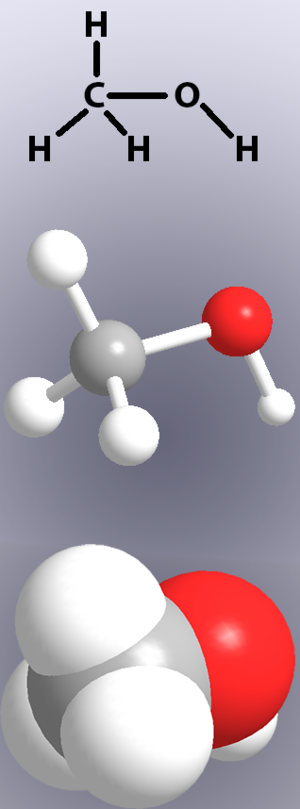
Chemical Bond - Energy Education
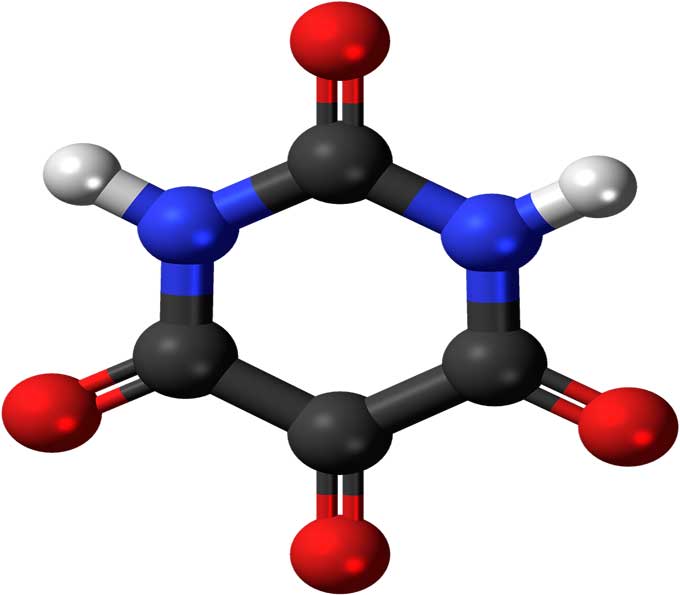
Chemical Bonding: (Types, Formation, And Facts) - Science4Fun
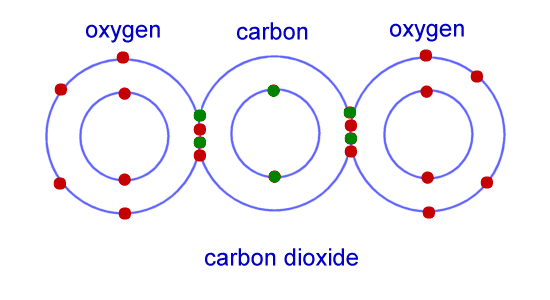
Chemistry For Kids: Chemical Bonding
/GettyImages-724235099-5a85a8c4119fa80037c0cb00.jpg)
Bonds Definition And Examples In Chemistry

Chemical Bonding Seventh Grade Science. Chemical Bonds Chemical Bonds Are The Glue That Holds The Atoms Of Elements Together In Compounds Chemical Bonds. - Ppt Download

Chemical Bonding | Definition, Types, & Examples | Britannica