The bond between a hydrogen atom and a chlorine atom in hydrogen chloride is formulated as follows: Covalent bonding is the result of.
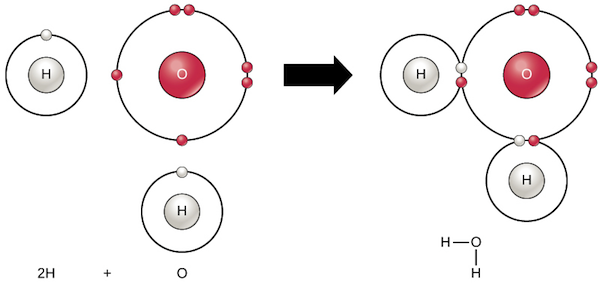
Chemical Bonds | Chemistry Of Life | Biology (Article) | Khan Academy
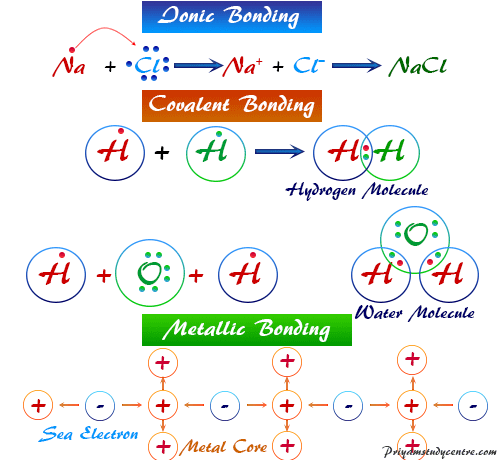
Covalent bonding is a common type of bonding in which two or more atoms share valence electrons more or less equally.

What is a covalent chemical bond. The simplest and most common type is a single bond in which. Covalent bonding, in simple words, is the sharing of electrons between atoms to attain the noble gas configuration of the participating individual atoms. A covalent bond is a bond where two or more atoms share electrons.
A covalent bond, or molecular bond, is a chemical bond formed between two atoms that share a pair of electrons; Commonly, carbon forms these types of chemical bonding. A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms.
This is because the atoms only share their outermost. For many molecules, the sharing of electrons allows each atom to attain the equivalent. The covalent bond is a type of chemical bond between the atoms of the same or different elements by the mutual sharing of pairs of electrons.
In lewis terms a covalent bond is a shared electron pair. When an atom interacts with another atom, it forms what is called a chemical bond. A covalent bond is a chemical bond formed by shared electrons.
Covalent bonds are formed between atoms by sharing electrons. The sharing of atoms helps complete the outer shell, or valence shell, of both atoms. Covalent bond in simple terms, a covalent bond is the exchanging of electrons between particles to achieve the honourable gas configuration of individual iotas.
These bonds are oriented in definite directions in. In a lewis structure of a. These electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding.
Covalent bonding a type of chemical bonding in which electrons are shared between atoms in a molecule or polyatomic ion., in. Valence electrons are shared when an atom needs electrons to complete its outer shell and can share those electrons with. A covalent bond is a type of chemical bond which is formed by the sharing of electrons between two or more atoms.
How do atoms share electrons in a covalent. There are three idealized types of bonding: In a covalent bond, the difference between the electronegativity of participating atoms is smaller than the ionic bonds.
A covalent bond is a chemical connection established between two atoms as a result of the mutual sharing of electrons. Covalent bonds are of particular importance in organic chemistry because of the ability of the carbon atom to form four covalent bonds. This interaction, this chemical bond links the two atoms together into something called a.
Covalent bonds are a type of chemical bonding that requires the sharing of electrons between atoms. There are several types of chemical formulas that you can use to represent chemical bonds.
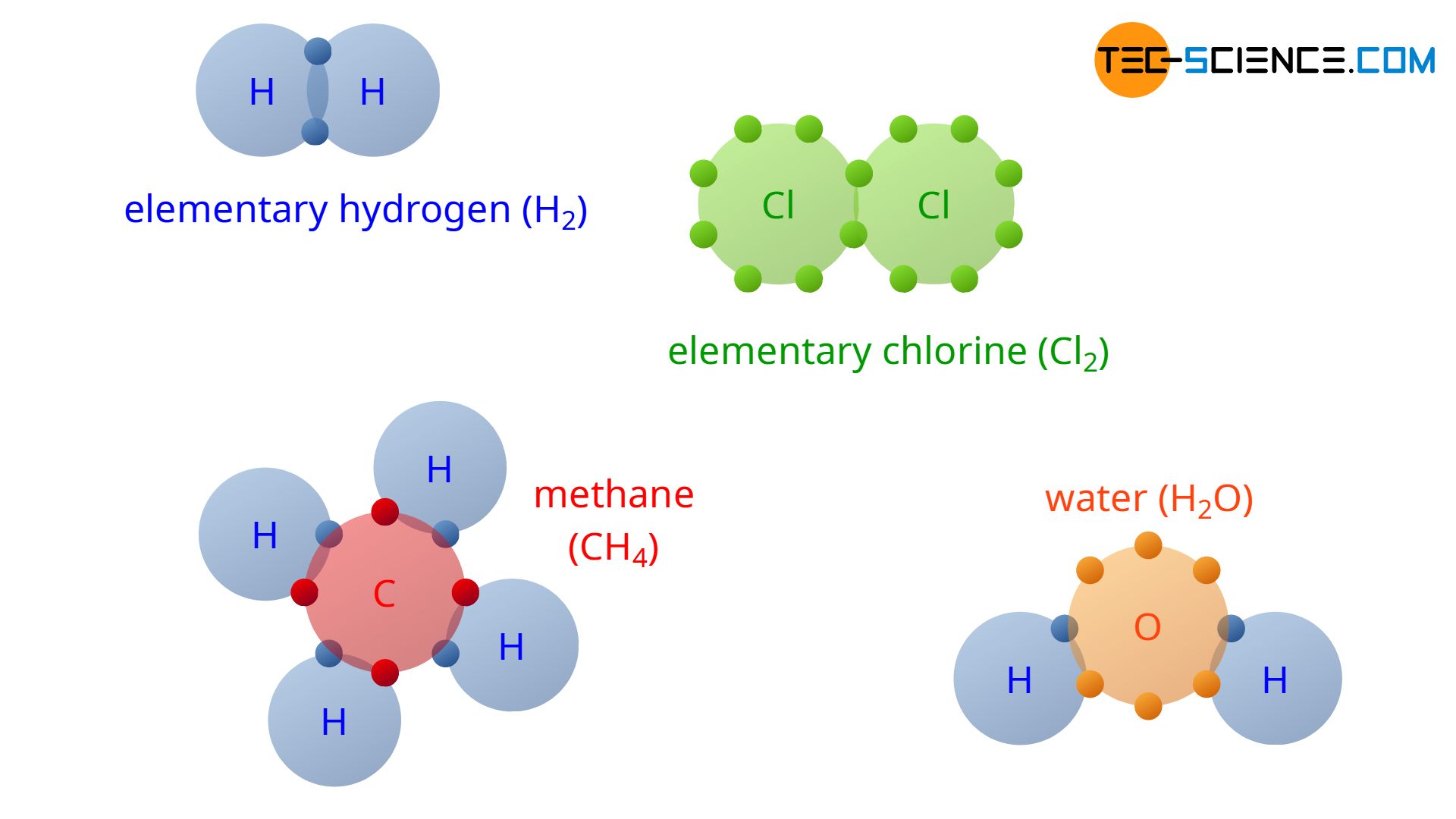
Covalent Bonding - Tec-Science
(85).jpg)
The Chemical Bond Test: Trivia Quiz - Proprofs Quiz
Chemical Bonds ( Read ) | Earth Science | Ck-12 Foundation

Ib Chemistry Standard Level Notes: Covalent Bonding

Covalent Bond: Definition, Types, And Examples
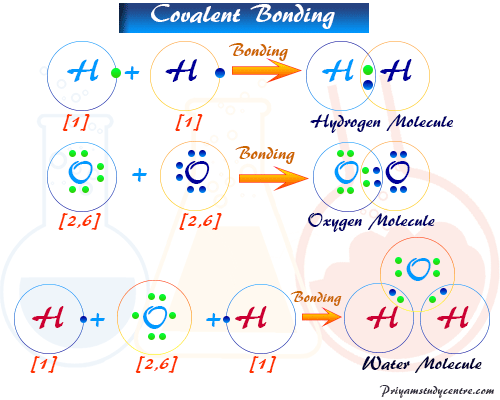
Covalent Bond - Types, Definition, Properties, Examples

Covalent Bond | Definition, Properties, Examples, & Facts | Britannica

Covalent Bond Definition And Examples - Biology Online Dictionary

Ionic Bonds Vs Covalent Bonds | Chemtalk
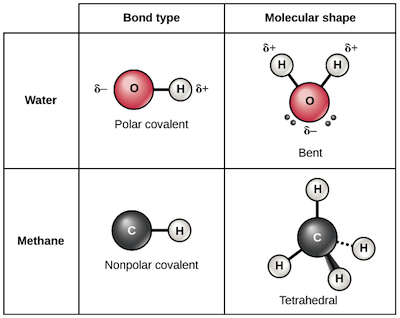
Chemical Bonds | Chemistry Of Life | Biology (Article) | Khan Academy

Chemical Bonds Iii: Polar Covalent - Video & Lesson Transcript | Study.com

Chemical Bonding - Covalent Bonds | Britannica

What Are Covalent Bonds Definition Examples And Types (Urdu /Hindi) | Chemistry | Chemical Bonding#3 - Youtube
What Are The Two Main Types Of Chemical Bonds? How Are They Formed? - Quora
Chemical Bonding - Definition, Types, Properties, Examples



