Common examples of solutions are sugar in water and salt. The solute is the substance that is.
Solution In Chemistry - Zoefact
In a chemical equation, the symbol (aq) follows a species name to indicate that it is in aqueous.
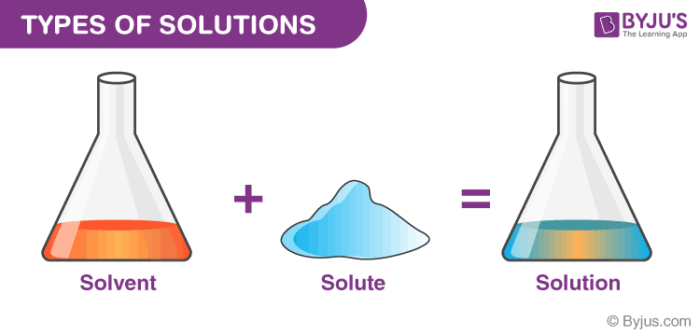
What is solution in chemistry. A solution consists of a solute and a solvent. A spiking solution is a standard that is chosen for preparing a matrix spike; A solution is a homogeneous mixture of two or more substances.
A solution is what occurs when two chemicals are mixed, referred to as a solvent and a solute. Water, concrete or gaseous may be a solution. A solution is a homogeneous mixture of two or more substances.
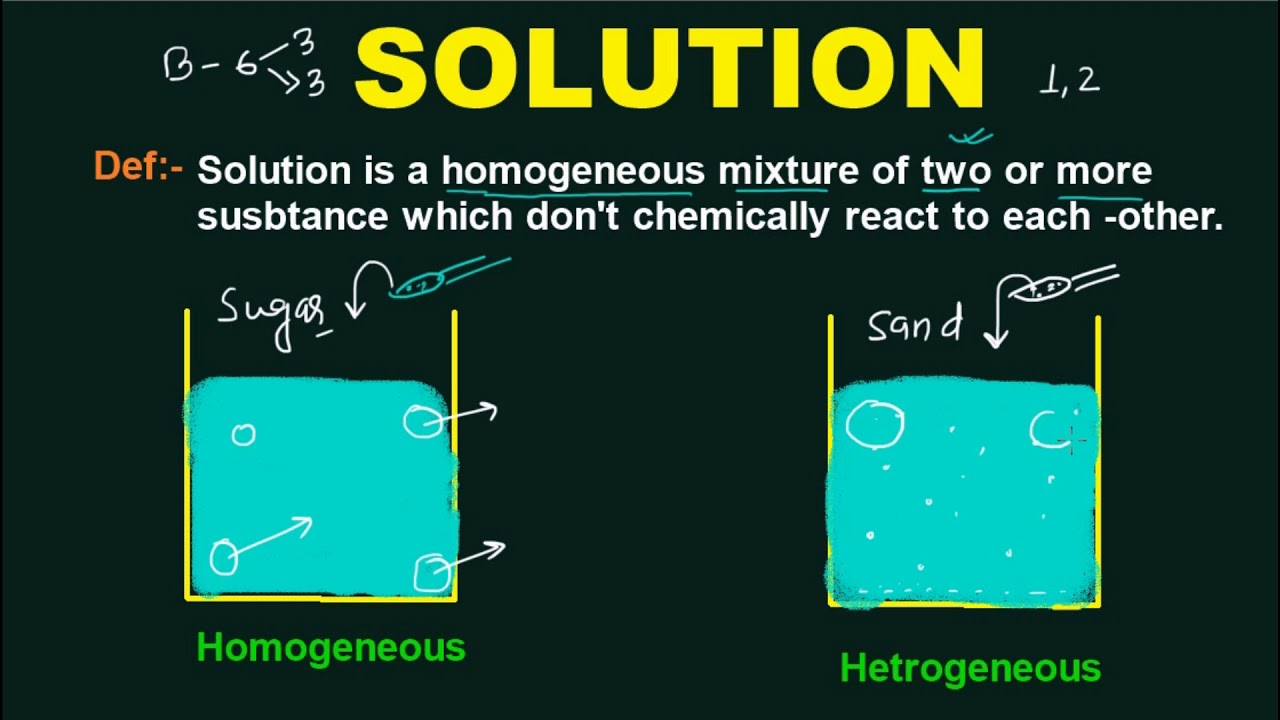
In chemistry, a solution is defined as a homogenous mixture of two or more compounds, where one compound is the solvent, and the other compounds are solutes. Homogeneous means that the components of the mixture form a single phase. Molarity of solution is defined as number of moles of solute dissolved per litre of.
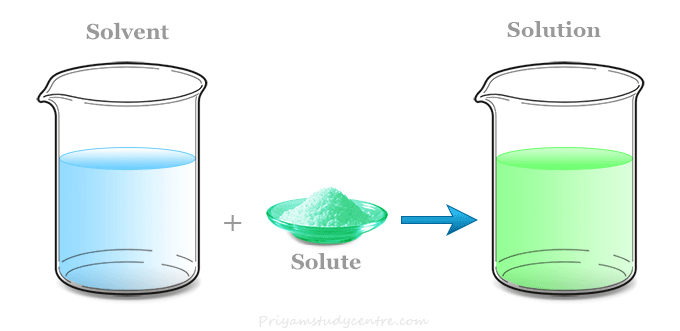
The combination of solute and solvent together is a solution. In comparison, a combination of liquids , gases and solids may. A solution does not allow.
The solute is the component of the solution that is being dissolved, and the solvent is the component of the. The solution, in chemistry, is a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to what is called the limit of solubility. Whether solids, liquids, or gases, solution chemistry is.
A solution in science is a homogenous mixture of two or more substances. A solution is a homogeneous mixture of one or more solutes dissolved in a solvent. The particles of solute in a solution cannot be seen by the naked eye.
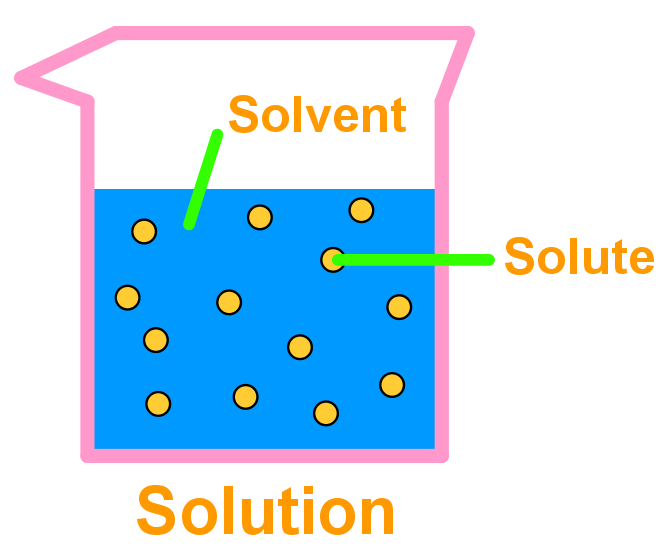
A solution may exist in any phase. The interface or surface is represented by separating the bulk phases by a hyphen. A solution is a combination of liquid and solute molecules that is homogeneous.
A solution is a homogeneous mixture of two or more components in which the particle size is smaller than 1 nm. The what is a solution in chemistry, it is no question simple then, previously currently we extend the connect to buy and make bargains to download and install what is a solution in chemistry. Solutions appear to be one substance, but the parts of a solution are not chemically bonded.
Solutions are uniform mixtures of molecules in which any of the phases of matter can be dissolved in another phase. An aqueous solution is any solution in which water (h 2 o) is the solvent. A solution, in science, refers to a type of mixture involving two or more substances.
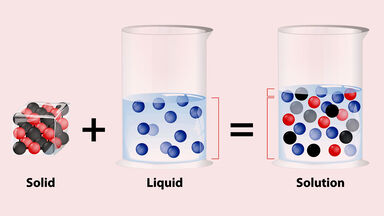
Solutions are a homogeneous mixture composed of a solute and a solvent. Solution, in chemistry, a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to what is called the limit of solubility. The substance in which a solute dissolves to produce a homogeneous mixture.
Surface chemistry deals with phenomena that occur at the surfaces or interfaces.

Solution - Definition, Properties, Types, Videos & Examples Of Solution.

18- Introduction To Solutions (1St Year Secondary First Term) - Youtube

Solution Chemistry Vocabulary Refresher: - Ppt Video Online Download
Solution - Definition, Types, Example - Chemistry
Introduction To Solution Chemistry And Solubility | Studypug
Common Examples Of Solutions: Science In Everyday Life

What Is A Solution In Chemistry? | Chemtalk
Solution Chemistry In Hindi L-1| Definition + Solution Chemistry Meaning,| | Eminent Guide - Youtube

Unsaturated Solution Definition And Examples In Chemistry
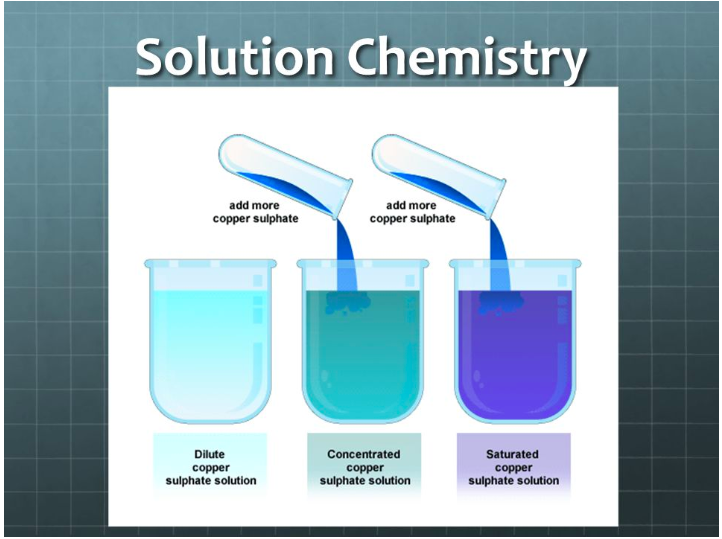
G8 Solution Chemistry - The!Mad!Scientist!

Solution Definition In Chemistry

What Is A Solution In Science? | Examples Of Solutions In Chemistry - Video & Lesson Transcript | Study.com
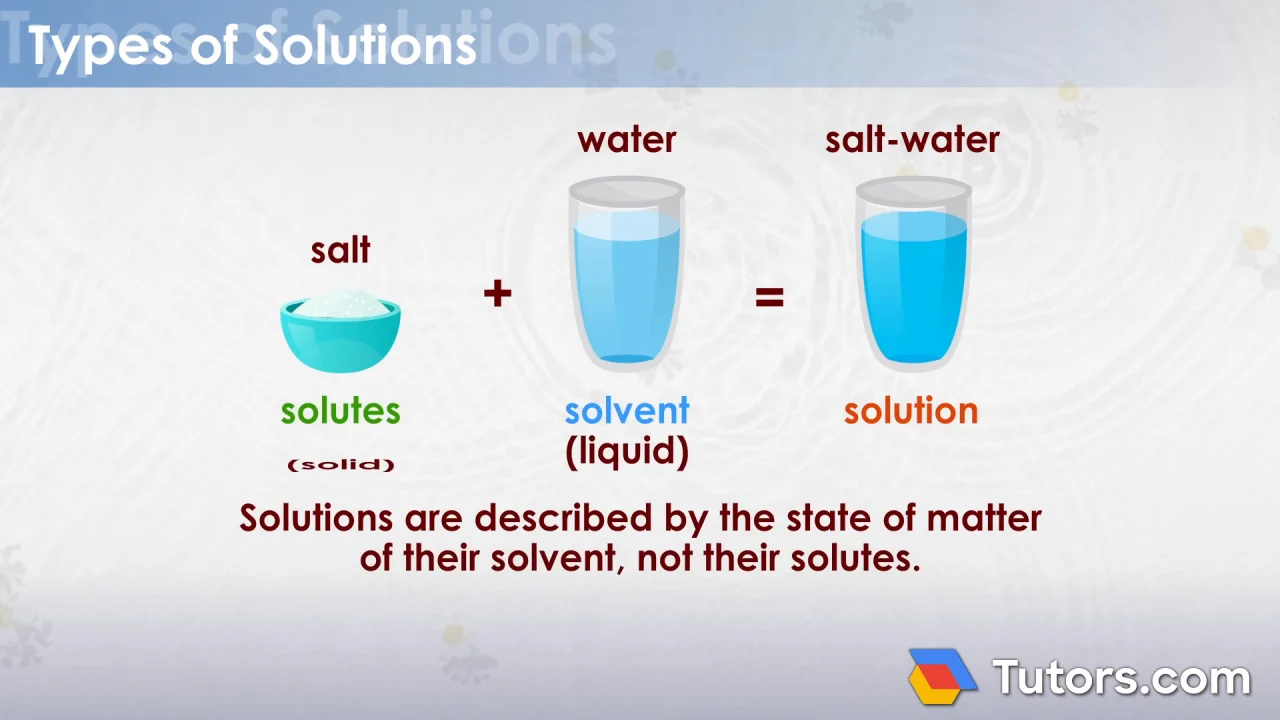
Solution | Chemistry Definition, Types, Examples (Tutors.com)
/79588918-56a1323e5f9b58b7d0bcf3a1.jpg)
Solution Definition In Chemistry

What Is A Solution In Chemistry? | Chemtalk

Solution | Chemistry Definition, Types, Examples (Tutors.com)

Types Of Solutions - Different Types, Homogeneous & Heterogeneous Solution With Videos

