If we want to accelerate an object, then we must apply a force. Vibrational motion of atoms in a bond that are stimulated by.

Work And Energy Class 9 Notes - Chapter 11 Key Highlights
It is defined as the work needed to accelerate a body of a given mass from rest to its stated.
Definition of kinetic energy in chemistry. It is to be contrasted with thermodynamics, which deals with the. In science, the kinetic energy (ke) of an object is the energy that it has because of its movement. Kinetic energy is a property of a moving object or particle and depends not only on its motion but also on its mass.
Kinetic energy is the energy an object possesses because of its motion. In physics, the kinetic energy of an object is the energy that it possesses due to its motion. Remember, momentum, the product of mass and.
Plug in all of the terms into the kinetic energy formula: This includes the analysis of conditions that affect speed of a chemical reaction, understanding. What is kinetic energy in chemistry?
Kinetic energy is essential in understanding the flow and the heating of liquids in. Kinetic energy is the same in chemistry as in physics — the energy an object possesses while it is in motion. The meaning of kinetic energy is energy associated with motion.
By definition, chemical energy is the ability of some substances to combine with others to develop energy in the form of light, heat, and electricity. An object in motion has the ability to do work and thus can be said to have energy. Identify the type of energy described by each of the following statements as potential or kinetic.
The kind of motion may be translation (or motion along a path. The kinetic energy definition in physics is given as: That sounds a bit complex,.
Kinetic energy is a scalar quantity, and it is. Chemical energy is energy stored in atoms. Kinetic energy of an object is the measure of the work an object can do by virtue of its motion.
It also deals with investigation of reaction mechanisms, the conditions of. Applying a force requires us to do work. Chemical kinetics is the study of chemical processes and rates of reactions.
Chemical kinetics, the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. 100 k g * ( 25 m / s) 2 2 = 62, 500 k g m 2 / s 2 2 = 31, 250 j. It is the work that is expected to quicken a body of a given mass from rest to its expressed.
Kinetic energy equation derived for a body can be applied to liquid particles to understand fluid mechanics. Kinetic energy is defined as the energy that is produced by an object due to its motion. This energy is dependent on the velocity of the object squared.
Kinetic energy is the energy an object has because of its motion. So, when the velocity doubles, consequently the kinetic. K = m v 2 2.
When an object is set to acceleration, there is a definite need to apply certain forces. The kinetic energy, k, is defined as the energy stored in an object because of its motion.

Kinetic Energy - Geeksforgeeks
Molecular Formulas And Nomenclature
/main-energy-forms-and-examples-609254-v3-5b562a0cc9e77c0037514831.png)
10 Types Of Energy And Examples
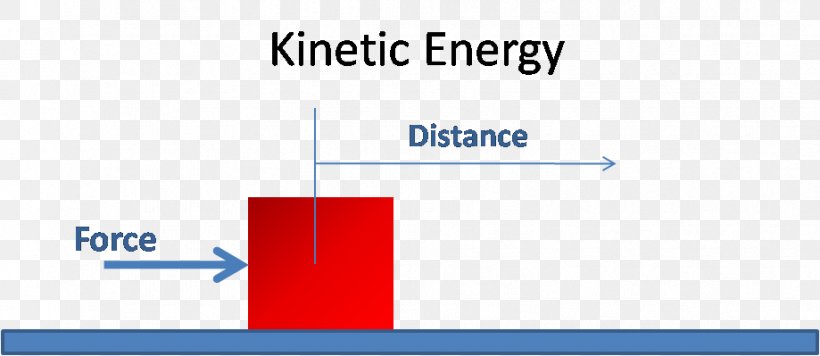
Kinetic Energy Potential Energy Definition Chemical Potential, Png, 919X399Px, Energy, Area, Blue, Brand, Chemical Potential Download

Kinetic Energy: Definition, Calculation, Types And Examples

Kinetic Energy: Definition, Formula & Facts - Eschool

What Is Kinetic Energy? Kinetic Energy Examples

What Is Kinetic Energy? Kinetic Energy Examples
Kinetic Energy In Chemistry Study Guide | Inspirit

What Is Chemical Energy? Definition And Examples

What Is Chemical Energy? | Chemical Energy Examples - Video & Lesson Transcript | Study.com

Potential And Kinetic Energy Explained
6.2: The Nature Of Energy - Chemistry Libretexts
P3. Energy, Work & Power - Mr. Tremblay's Class Site

Kinetic And Potential Energy. Potential Energy Is That Energy Which An Object Has Because Of Its Position. It Is Called Potential Energy Because It Has. - Ppt Download
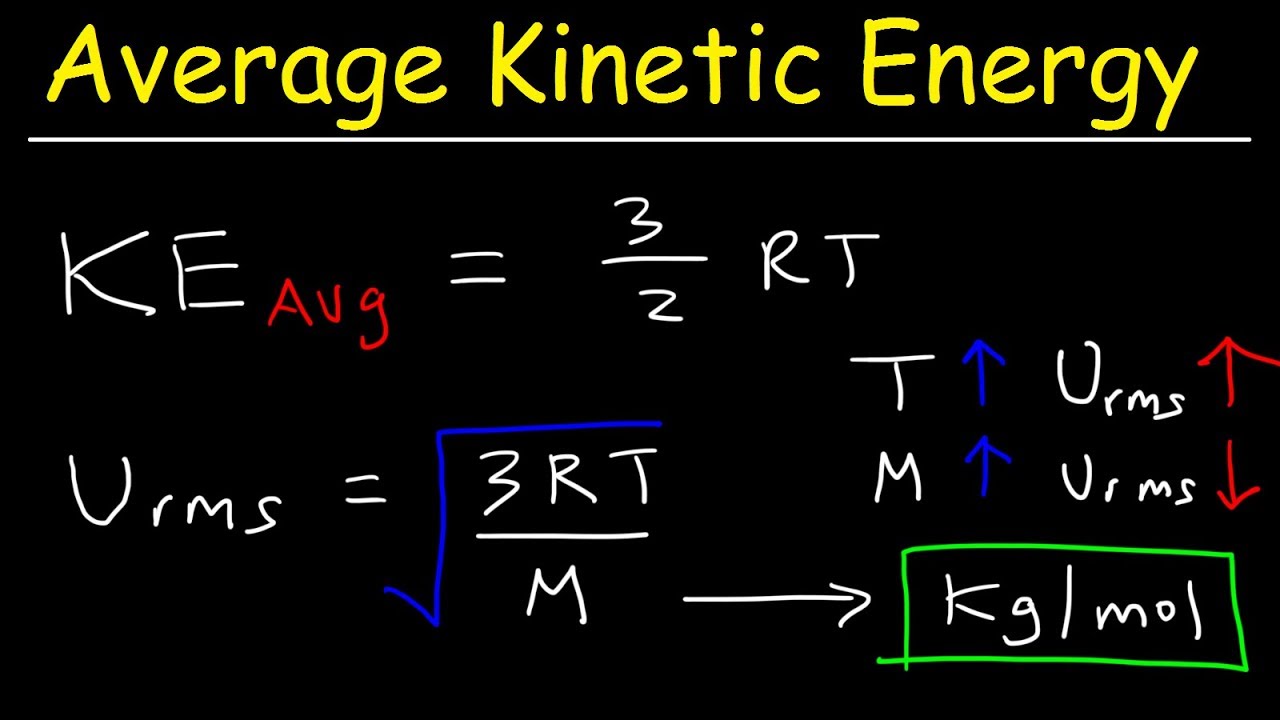
Average Kinetic Energy Of A Gas And Root Mean Square Velocity Practice Problems - Chemistry Gas Laws - Youtube

Kinetic Energy - Definition, Formula, Examples - Teachoo

Kinetic Potential Chemical Thermal Definition Energy Of Motion - Ppt Download
/85375779-56a12f273df78cf7726837cc.jpg)