This can be used to classify reactions as exothermic or. Here are some typical examples of exothermic and endothermic reactions.

Exothermic Reaction Concept & Examples | What Is An Exothermic Reaction? - Video & Lesson Transcript | Study.com
Endothermic reactions, the opposite of exothermic, is a chemical reaction in which heat energy is absorbed/taken in.

Examples of exothermic energy in chemistry. In chemistry, endothermic reaction is defined as one type of reaction in which any system absorbs energy in form of heat, light from surroundings. Another way to think of exothermic versus endothermic reaction is by. Energy profile and activation energy.
Endothermic reactions are a result of bonds being broken, which requires. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Aerobic and anaerobic respiration occurs in mitochondria in a cell and generates heat energy to help in different biological activities in living organism.
The burning of fuel is an example of a combustion reaction, and we as humans rely heavily on this process for our energy requirements. You can think about this visually using a reaction energy diagram,. Exothermic reactions release energy to their surroundings, because the products are lower in energy than the reactants.
Examples of endothermic reactions include photosynthesis (which uses sunlight) and melting ice cubes (which uses heat). Exothermic meaning is a chemical reaction that involves the release of energy in the form of heat or light is known as an. Can you think of a.
Matching a light using a matchstick is one. In an exothermic reaction, heat is released from the reacting chemicals. Exothermic reactions release energy into the surroundings, so they usually feel hot.
An exothermic reaction is one in which. Examples of exothermic and endothermic reactions. An endothermic process absorbs heat and cools the surroundings.
Learn more in this ks3 chemistry guide from bitesize. In an exothermic reaction, the energy of the products. Investigating temp changes copper sulfate.
Endothermic reactions are the opposite. The rusting of steel is an example of an exothermic chemical reaction. The following equations describe the combustion of a.
So, in the list of exothermic. Sometimes, energy is absorbed during a chemical reaction. An exothermic process releases heat, and causes the temperature of the immediate surroundings to rise.
Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. An exothermic reaction is a chemical reaction that releases energy by light or heat the diagram above outlines the energy reaction diagram for an exothermic reaction. A reaction that is chemical in nature and is characterized by the release of energy in the form of heat or light is called an exothermic reaction.
A good example of an endothermic reaction is photosynthesis. Whereas formation of anion is an. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products.
This page gives a number of examples of simple exothermic reactions. In reactions that absorb energy, the reactants are more stable, and their bonds have less energy than those of the.

Exothermic Reaction: Definition, Equation, And Examples

What Are Endothermic & Exothermic Reactions | Chemistry | Fuseschool - Youtube
Endothermic Vs. Exothermic Reactions (Article) | Khan Academy

Endothermic Vs Exothermic Reactions | Chemtalk
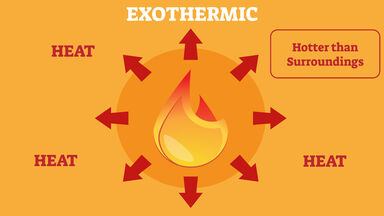
Exothermic Reaction Examples Found In Real Life
Endothermic Vs. Exothermic Reactions (Article) | Khan Academy

Exothermic Reaction: Definition, Equation, And Examples

Difference Between Endothermic And Exothermic Reactions| Chemistry
/endothermic-and-exothermic-reactions-602105_final-c4fdc462eb654ed09b542da86fd447e2.png)
Endothermic And Exothermic Chemical Reactions

Exothermic Vs. Endothermic: Chemistry's Give And Take - Discovery Express

Endothermic Reactions - Definition And Examples

Exothermic Reaction - Wikipedia
Exothermic Reaction | Ck-12 Foundation

Exothermic Reactions - Definition And Examples
/endothermic-and-exothermic-reactions-602105_final-c4fdc462eb654ed09b542da86fd447e2.png)
Endothermic And Exothermic Chemical Reactions

Exothermic Reaction: Definition, Equation, And Examples
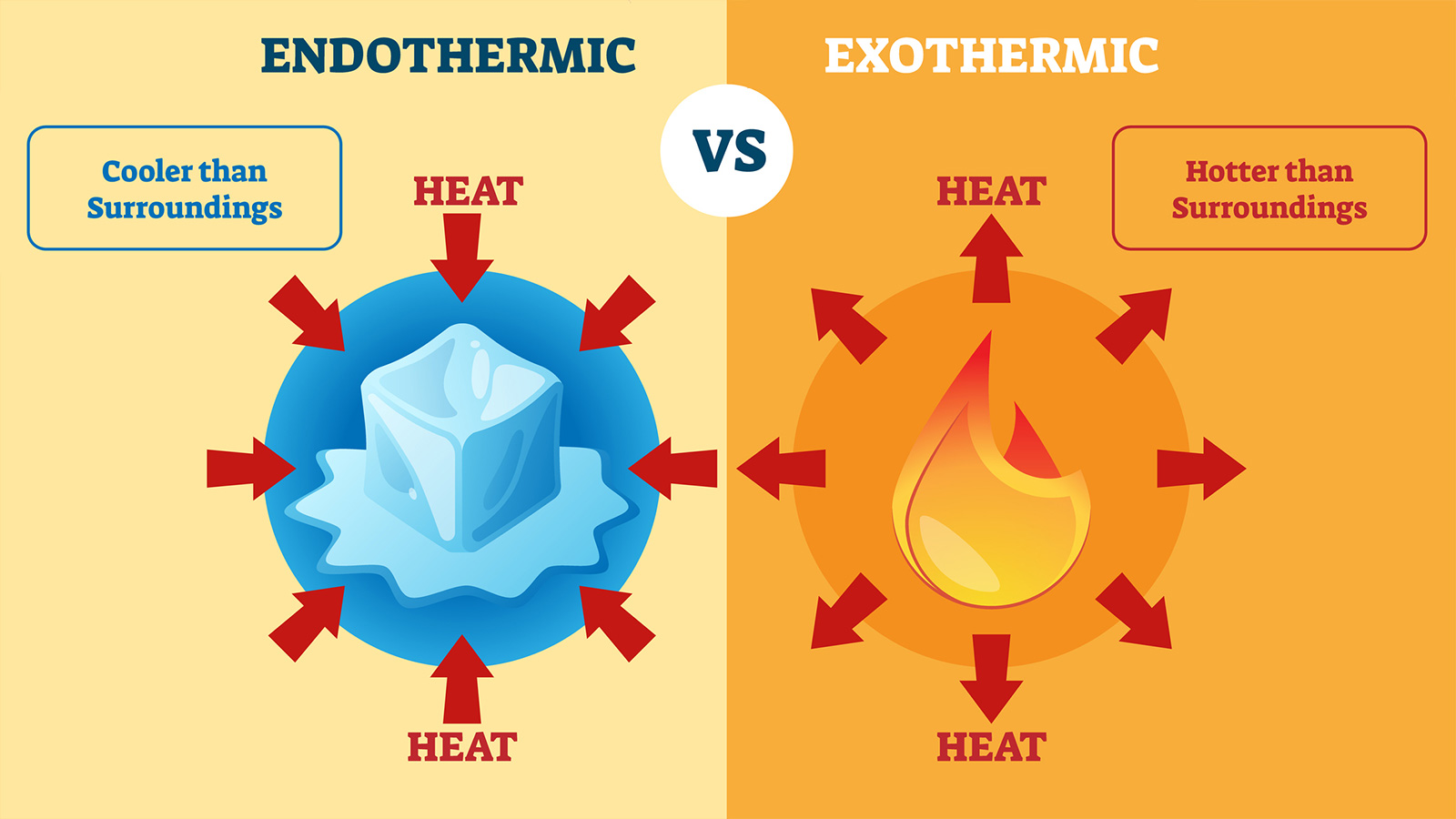
Simple Endothermic Reaction Examples

Enthalpy: Energy Transfer In Physical And Chemical Processes - Video & Lesson Transcript | Study.com
Endothermic Vs. Exothermic Reactions (Article) | Khan Academy


