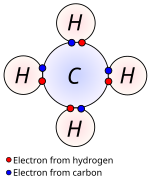
Bonding is the combination of two or more elements either by transfer of electrons or sharing in order to attain a stable configuration. Bond order is a measurement of the number of electrons involved in bonds between two atoms in a molecule.
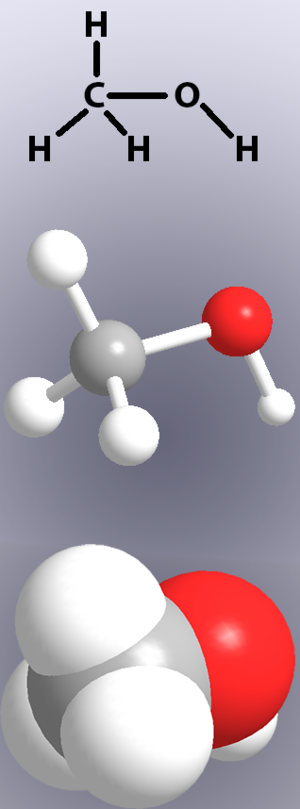
Chemical Bond - Energy Education
Three idealized types of bonding are ionic bonding , in which.

What is a bond in chemistry. It is used as an indicator of the stability of a chemical bond. Once joined, two or more. There are basically two types of bonding.
This attraction may be seen as the result of different behaviors of the outermost or valence electrons of atoms. A type of chemical bond formation which occurs because of the transfer of electrons from one atom. The nature of the other bonds in the molecule influences the exact parameters of a certain form of bond;
This force is due to the interaction between the atoms of the molecule. This force is known as a chemical bond. Learn about different forms of chemical bonding, and how those bonds are broken, in this tutorial.
Chemical bonding is the general term used to describe the forces that hold atoms together in molecules and ions. It is also called an electrovalent bond. Atoms can come together by exchanging electrons to form molecules.
This bonding will also create an anti bonding orbital. But not only have these distinctions. Definition of bond there is a chemical bond between two atoms or groups of atoms in case that the forces acting between them are such as to lead to the formation of an aggregate with.
Thus to conclude, single bonds between atoms have a sigma. An atom that has gained an electron has a negative charged and is called an anion. Once this orbital is filled, the bond between the 2 atoms breaks.
Chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other stable species that make up the familiar substances of the. Chemical bonding is the combination of atoms , molecules or ions that form more complex and stable compounds, altering their physical and chemical properties. Chemical bonding is the process of uniting two or more atoms by the redistribution of electrons, resulting in each atom achieving a stable electronic state.
When the transfer of electrons takes place, a. The term ionic means the electrical pull between positive and negative ions. The bond energy is the sum of a molecule's bond dissociation energies.
The phenomenon of the union of two or more atoms involving the redistribution of electrons so that each atom involved in bonding acquires a. A chemical bond is an attraction between atoms. Molecules of chemical substances are joined together by some force.
The chemical bond which is formed by the transfer of electrons between atoms is known as an ionic bond.

Chemical Bonding | Definition, Types, & Examples | Britannica

Covalent Bond | Definition, Properties, Examples, & Facts | Britannica
/GettyImages-724235099-5a85a8c4119fa80037c0cb00.jpg)
Bonds Definition And Examples In Chemistry

Chemical Bonds: Definition, Types, And Examples

Chemical Bonding - Ionic Vs. Covalent Bonds - Youtube
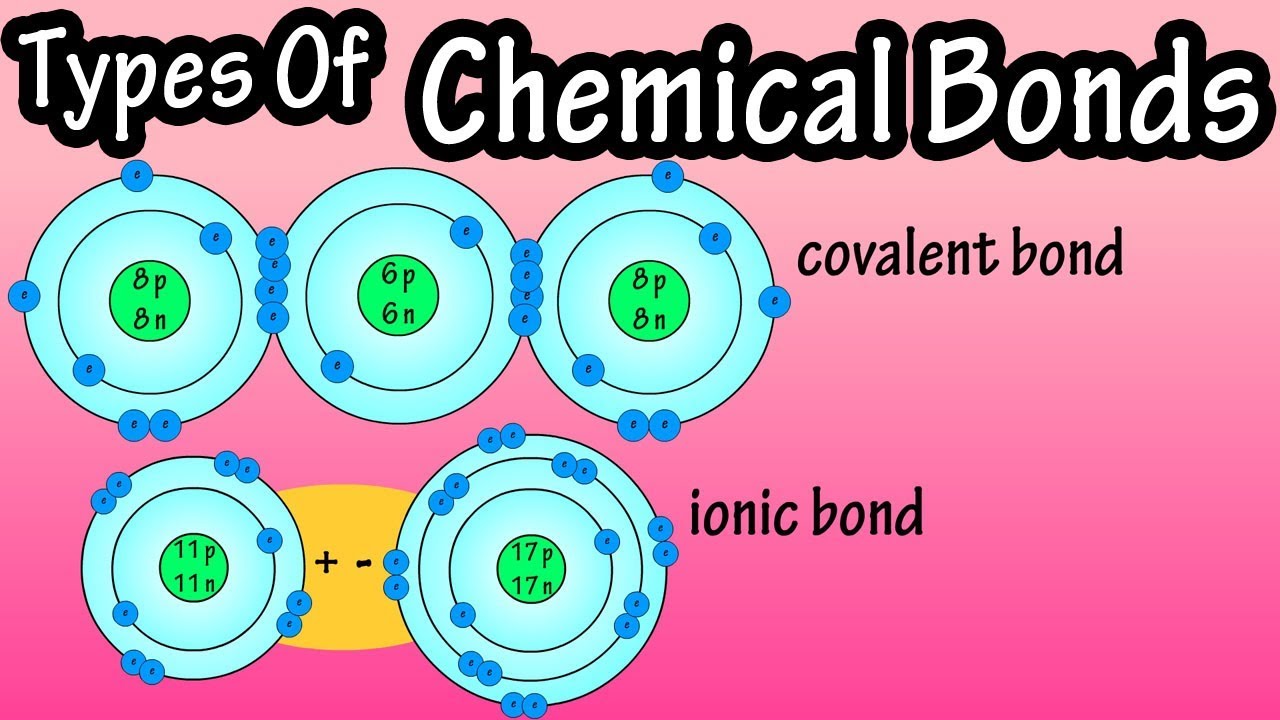
Types Of Chemical Bonds - What Are Chemical Bonds - Covalent Bonds And Ionic Bonds - What Are Ions - Youtube

Chemical Bonding | Definition, Types, & Examples | Britannica
Chemical Bonding: (Types, Formation, And Facts) - Science4Fun
Chemical Bond - Examples, Body, Used, Water, Type, Form, Energy, System, Oxygen
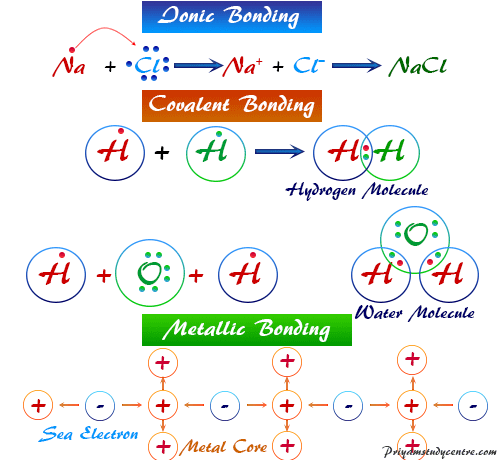
Chemical Bonding - Definition, Types, Properties, Examples
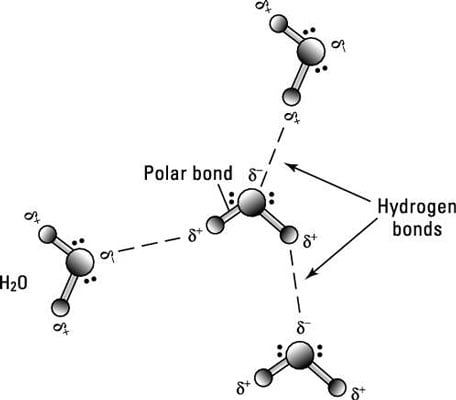
4 Types Of Chemical Bonds - Dummies
(85).jpg)
The Chemical Bond Test: Trivia Quiz - Proprofs Quiz
What Is A Chemical Bond? - Quora
What Is A Bond? | Feature | Chemistry World
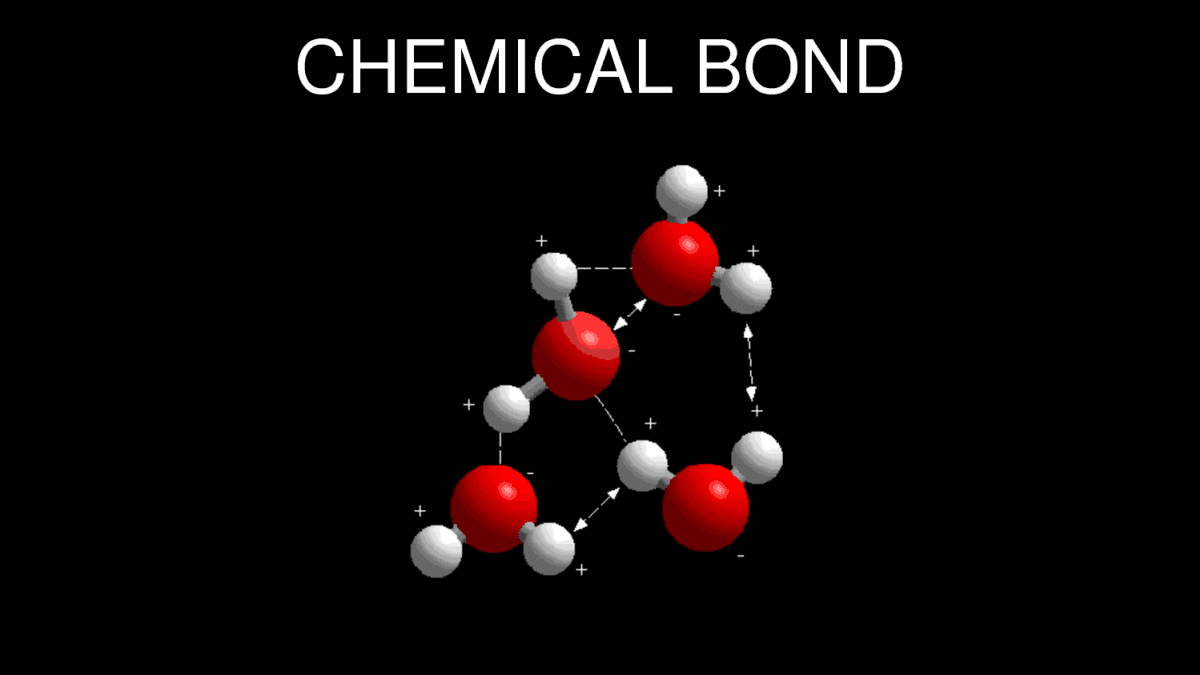
Chemical Bonding: How Do Atoms Combine? What Are The Forces That Bind The Atoms Together? - Owlcation